Tisotumab vedotin
Overview + Rationale
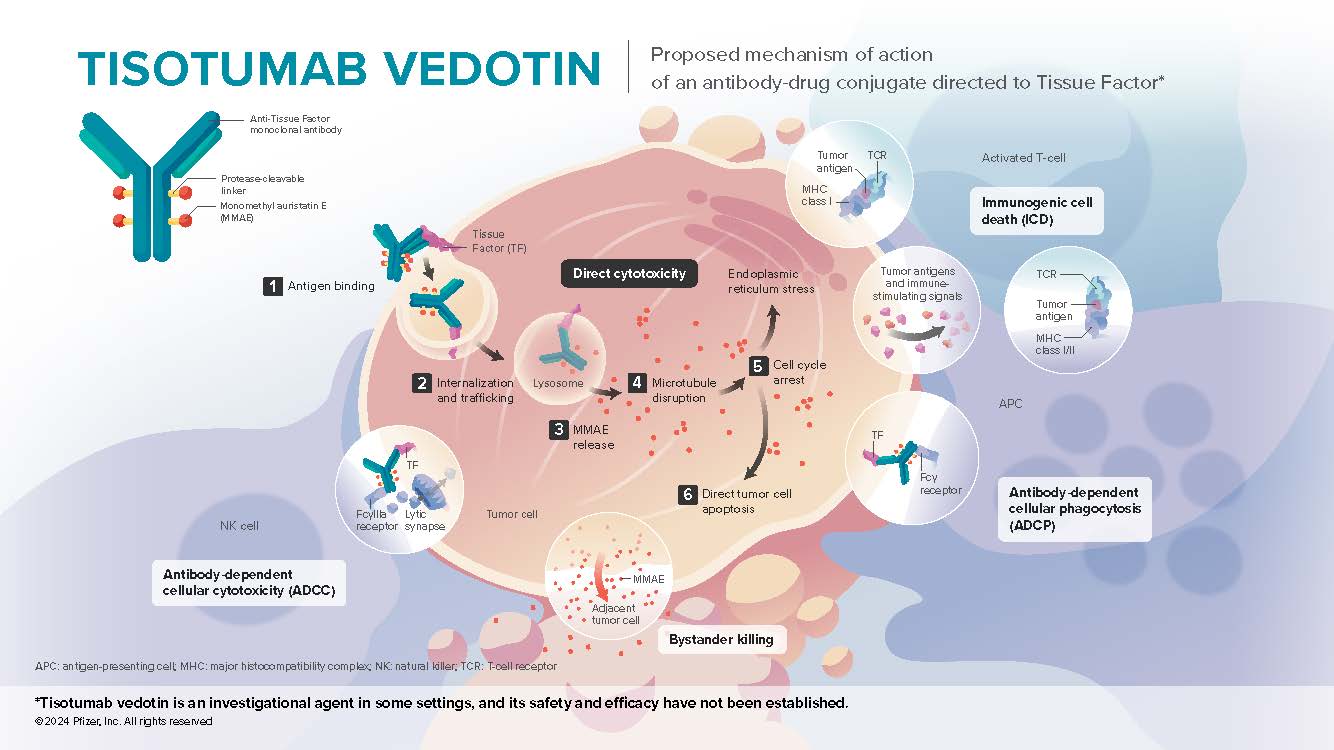
- Tisotumab vedotin is an antibody-drug conjugate directed to tissue factor (TF), a protein that is highly prevalent in cervical cancer and many solid tumors. TF promotes cancer growth and metastasis and is associated with poor prognosis1-4
- In normal physiology, TF initiates the coagulation cascade after vascular injury5,6
- In tumor cells, TF has been shown to promote tumor growth, angiogenesis, and metastasis5,6 TF is prevalent in several solid tumors, including cervical cancer and head and neck cancers7,8
- In these tumors, where TF is present, levels are elevated relative to normal tissue 2,4
Partner: Genmab A/S
Mechanism of Action
Stage of Development
Tisotumab vedotin is being investigated in the tumor types shown below. Safety and efficacy for the use listed below have not been established.
Advanced Solid Tumors
Recurrent or Metastatic Cervical Cancer
Phase 3 Monotherapy*

 Back
Back
