Maplirpacept (TTI-622 | PF-07901801)
Maplirpacept (TTI-622 | PF-07901801) is an investigational compound. Its safety and efficacy have not been established.
Overview + Rationale
RATIONALE FOR CANCER TARGET
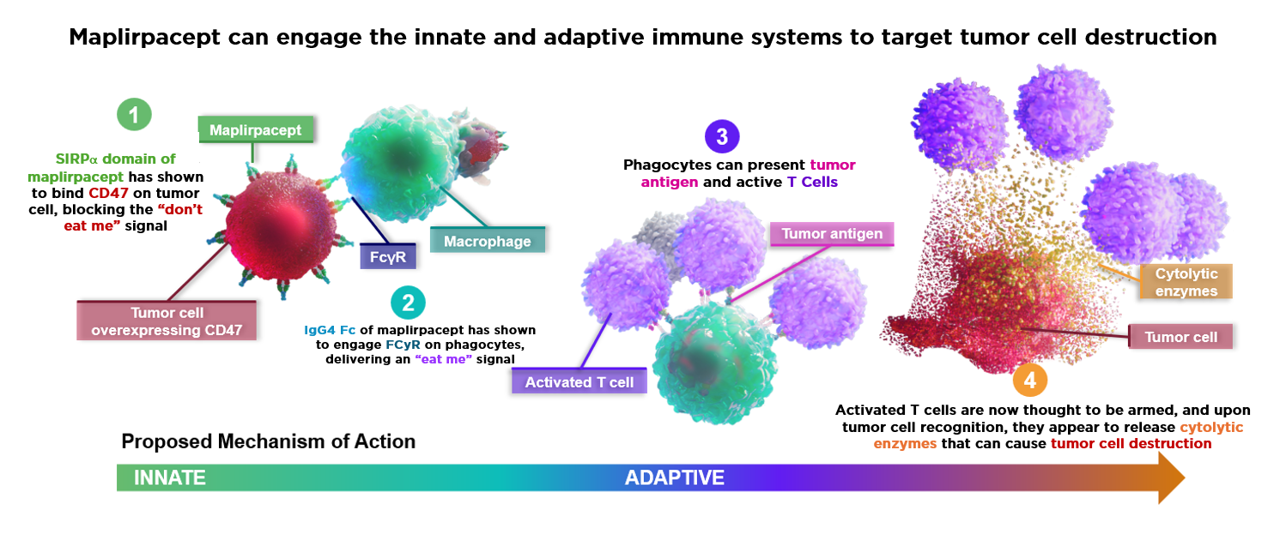
- CD47, or integrin-associated protein, is a cell surface ligand expressed in low levels by nearly all cells of the body. It plays an integral role in various immune responses as well as autoimmunity, by sending a “don’t eat me” signal to prevent phagocytosis
- CD47 is overexpressed in various hematological malignancies and its interaction with Signal Regulatory Protein (SIRP)α on phagocytic cells prevents phagocytosis of cancer cells
- Additionally, it is expressed by different cell types in the tumor microenvironment and is required for establishing tumor metastasis. Overexpression of CD47 is thus often associated with poor clinical outcomes
- CD47 blockade promotes both innate (macrophage phagocytosis) and adaptive immunity (T cell responses)
OVERVIEW
- Maplirpacept (TTI-622 | PF-07901801) is a fusion protein designed to enhance phagocytosis and antitumor activity by preventing CD47 from delivering its inhibitory signal as well as generating a moderate pro-phagocytic signal via IgG4 Fc
- Maplirpacept minimally binds to human red blood cells
Mechanism of Action
Stage of Development
R/R Diffuse Large B-Cell Lymphoma (DLBCL) Transplant Ineligible
Phase 2 Combination*§
Multiple Myeloma
Phase 1 Combination†

 Back
Back
