CEACAM5-directed Antibody Drug Conjugate
PF-08046050 is an investigational compound. Its safety and efficacy have not been established.
Overview + Rationale
- A member of the CEACAM sub-group family of glycoproteins involved in cell adhesion.1
- Observed in tumors of epithelial origin such as CRC, PDAC, GC, and NSCLC.2
- Normally expressed during fetal development limited in adults.1,2
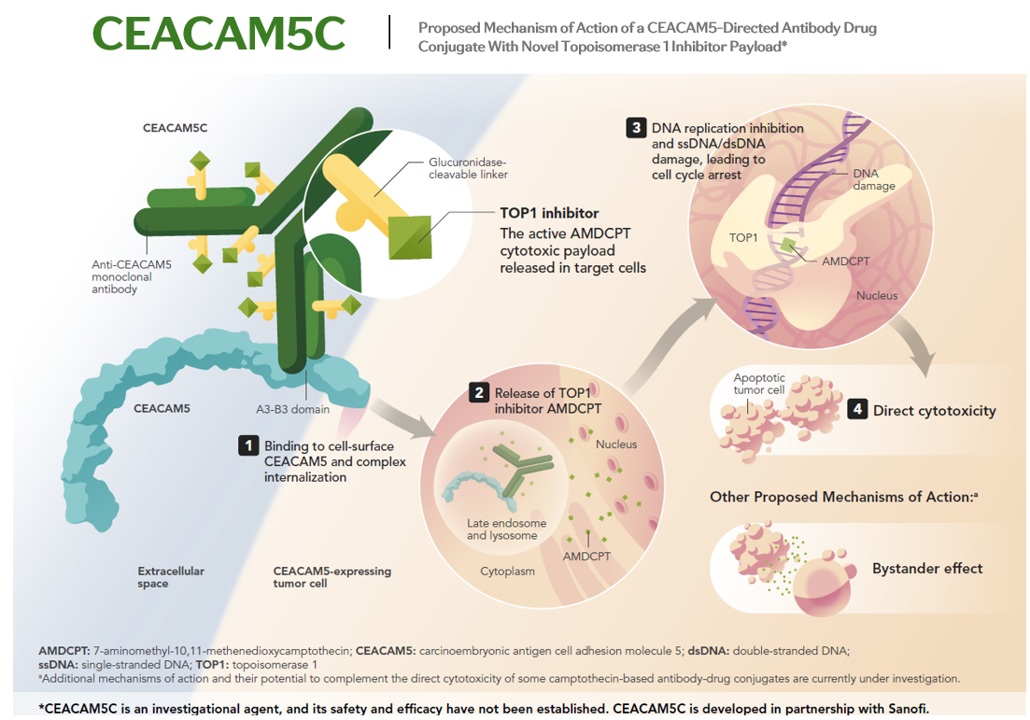
- CEACAM5C is an investigational antibody-drug conjugate, comprising a monoclonal antibody directed to CEACAM5 and a novel TOP1 inhibitor linker-payload system, which enables preferential release of a topoisomerase 1 inhibitor within target cells.3
Mechanism of Action

 Back
Back
