STING Agonist
PF-07820435 is an investigational compound. Its safety and efficacy have not been established.
Overview + Rationale
•Insufficient cytotoxic T lymphocyte infiltration and the accumulation of immunosuppressive cells within tumors are factors in the inadequate clinical effectiveness of cancer treatment1
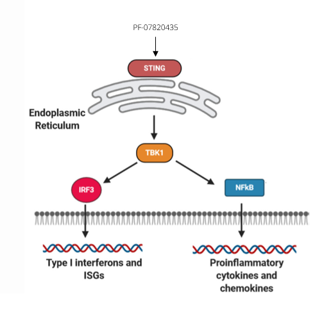
•The ‘stimulator of interferon genes’ (STING) is an endoplasmic protein that induces the production of pro-inflammatory cytokines such as type I interferons (IFNs)2
•The STING pathway drives activation of type I interferons and other inflammatory cytokines in the immune response against tumors2
•STING agonists lead to activation of downstream signaling via phosphorylation of TBK1 and IRF3 and induction of the type I IFN3
•PF-07820435 is an orally delivered STING agonist being developed as a type 1 IFN inducer for a broad range of solid tumors3
•In vivo, antitumor activity was shown following PF-07820435 treatment of tumor-bearing hSTING-KI mice3
Mechanism of Action
PF-07820435-mediated STING induction is intended to induce both adaptive and innate immune responses resulting in improved tumor control by
•Inducing immune-stimulatory cytokines, enhancing antigen presentation, and T-cell activation;
•Increasing T-cell infiltration in TME; and
•Eliciting tumor cell death via T cell and NK cell mediated tumor cell killing
Data suggest that PF-07820435 can elicit type I IFN responses in PBMCs, myeloid cells and multiple tumor cell types in people and cancer patients.

 Back
Back