MEK Brain Penetrant Inhibitor
PF-07799544 is an investigational compound. Its safety and efficacy have not been established.
Overview + Rationale
RATIONALE FOR CANCER TARGET
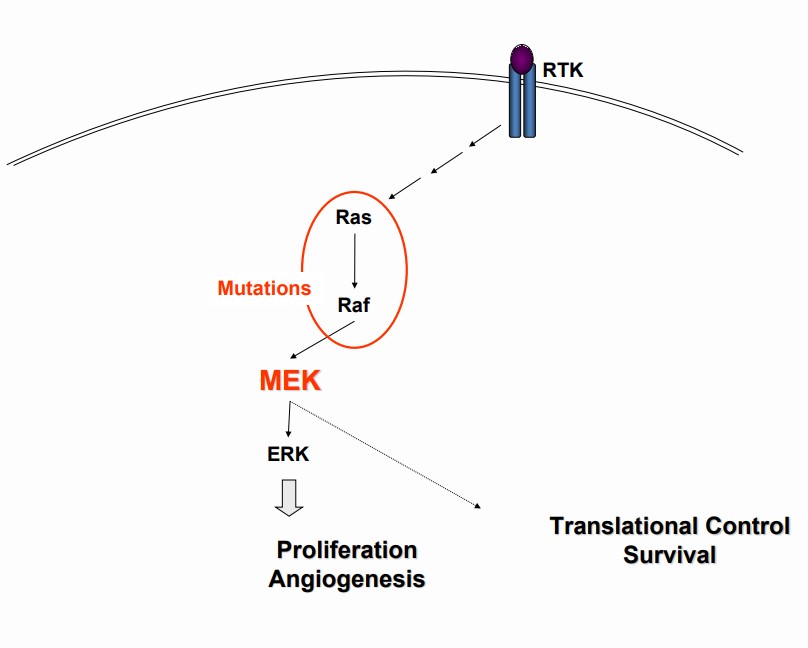
- The mitogen-activated extracellular signal regulated kinase 1 (MEK1) and MEK2 are upstream regulators of the extracellular signal-related kinase (ERK) pathway1
- MEK1/2 are dual-specificity threonine/tyrosine kinases that play roles in the activation of the RAS/RAF/MEK/ERK pathway and are often upregulated in a variety of tumor cell types2
- Current MEK inhibitors appear to be only modestly brain penetrant3
- Acquired MEK mutations account for resistance to approved BRAF/MEK inhibitor combinations (prevalence 7-10%)4
- Despite survival improvements in BRAF metastatic tumors with ICI & targeted therapy, brain metastases remain a major cause of morbidity and mortality3
- Drug delivery across the blood brain barrier (BBB) is a factor that requires attention to address this unmet need5
OVERVIEW
- PF-07799544 drives anti-tumor activity in BRAF-mutant xenograft models when in combination with BRAF inhibitors and shows drug exposure in the brain and activity in intracranial tumor models
- PF-07799544, in combination with the brain penetrant BRAF dimer selective inhibitor PF-07799933, has demonstrated anti-tumor activity in non-clinical models of intracranial disease that are driven by BRAF mutations and MAPK signaling
Mechanism of Action
- In pre-clinical models, PF-07799544 selectively binds and affects the MEK1/2 kinases, slowing both signal transduction and proliferation across MAPK pathway-driven cell lines
- PF-07799544 is a brain-penetrant, reversible inhibitor of mitogen-activated extracellular signal regulated kinase 1 (MEK1) and MEK2 activity
- In vitro, PF-07799544 showed activity related to both ERK phosphorylation and cellular proliferation across select cell lines harboring activating mutations in BRAF, KRAS, or NRAS
Inhibition

 Back
Back
