CD228V directed Antibody Drug Conjugate
PF-08046031 | CD228V is an investigational compound. Its safety and efficacy have not been established
Overview + Rationale
RATIONALE FOR CANCER TARGET
- CD228 is highly expressed in multiple MMAE-sensitive tumor types1
- CD228 has possible role in tumor proliferation and migration; limited expression in normal tissues by IHC
OVERVIEW2
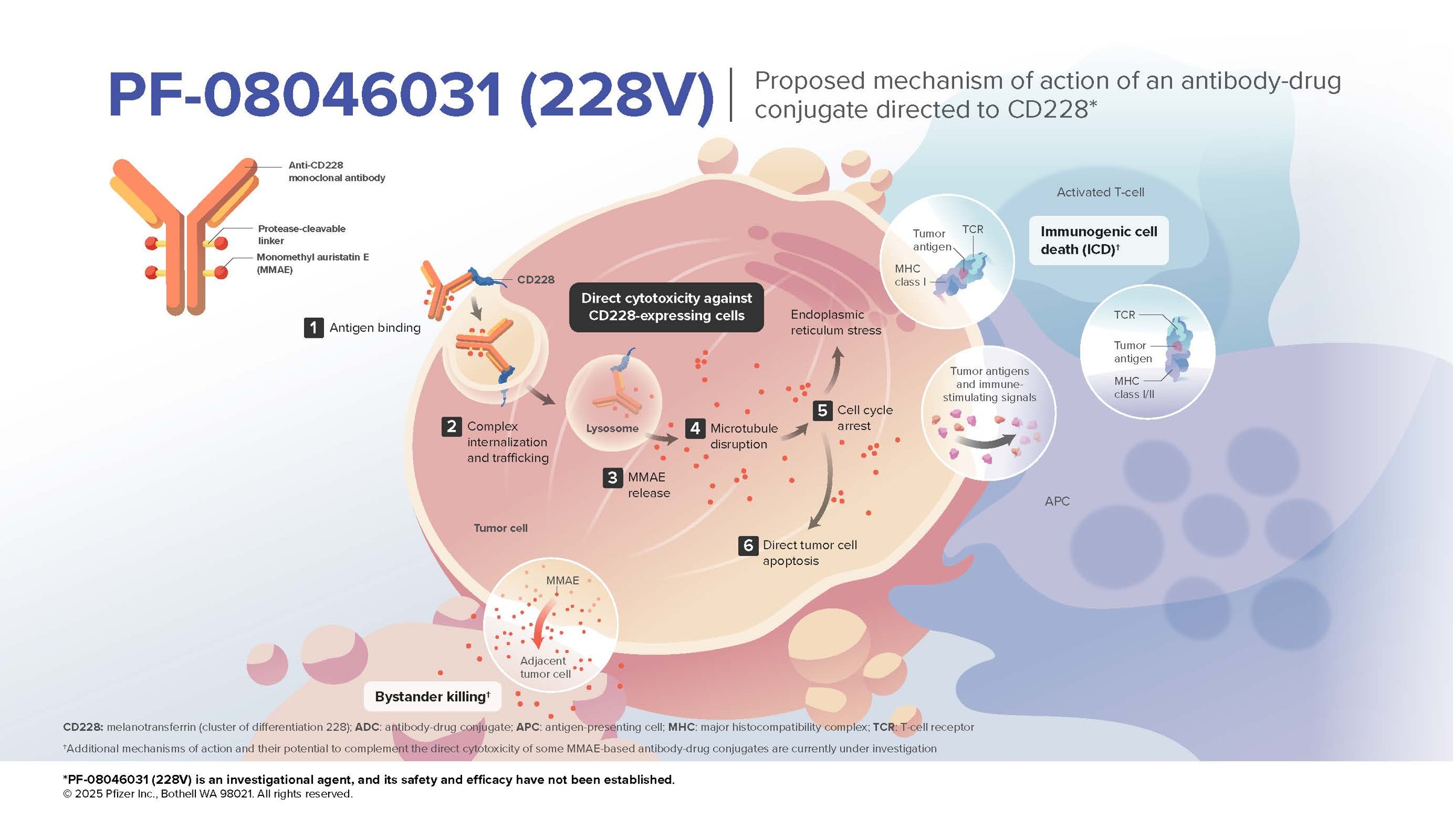
PF-08046031 is an investigational ADC composed of a humanized IgG1 anti-CD228 monoclonal antibody, hL49, conjugated to an average of 4 molecules of the microtubule-disrupting agent monomethyl auristatin E (MMAE) via a protease cleavable valine citrulline linker system (vedotin).
Mechanism of Action

 Back
Back

