Tucatinib
Overview + Rationale
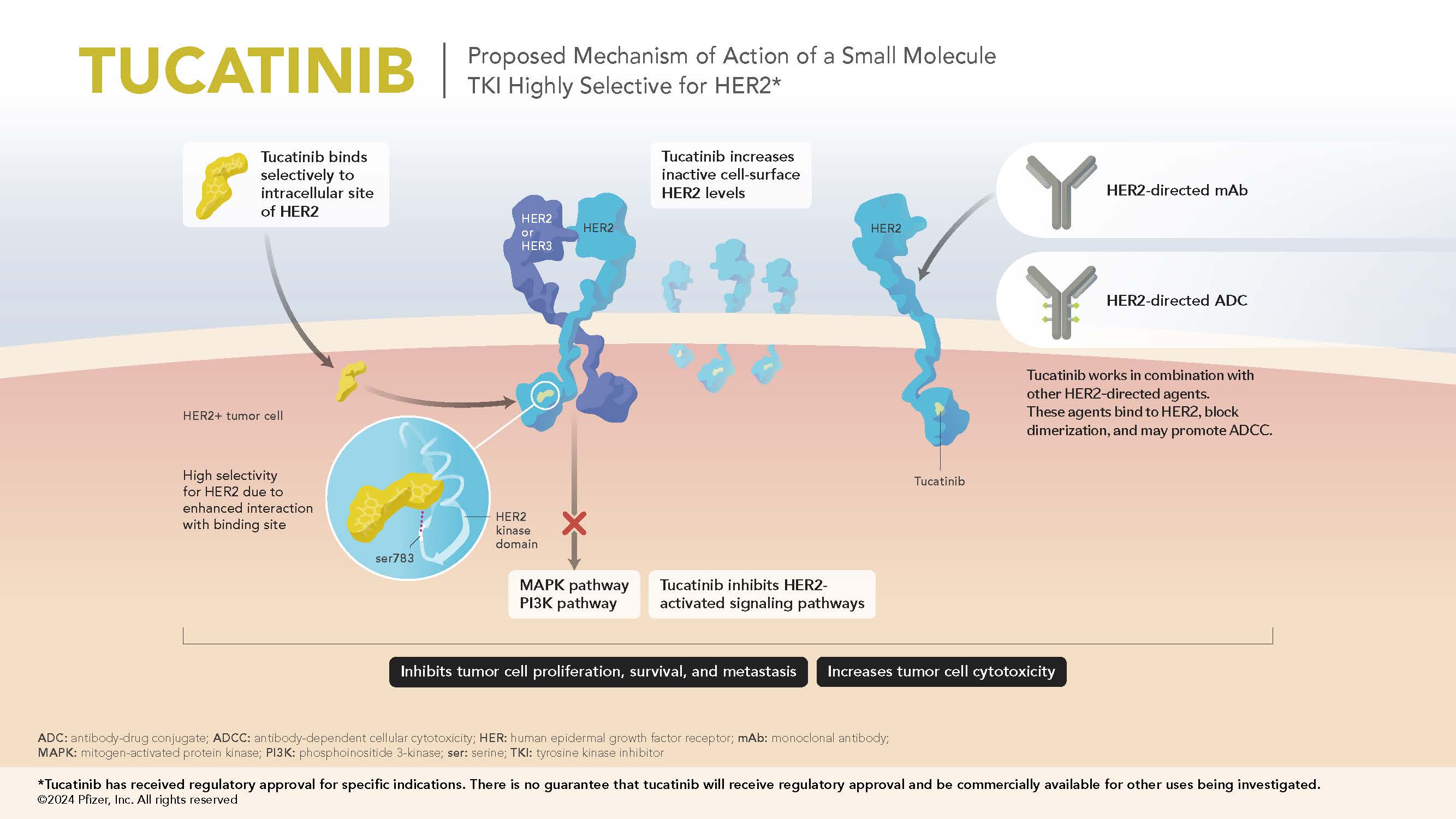
- Tucatinib is an orally bioavailable, reversible, highly selective small molecule tyrosine kinase inhibitor being investigated in multiple human epidermal growth factor receptor 2 (HER2) overexpressed/amplified and HER2 mutated cancers.1,2
- In cell signaling assays, tucatinib exhibits thousandfold greater selectivity for the HER2 receptor relative to epidermal growth factor receptor (EGFR).1
- HER2 selectivity may decrease the potential for EGFR-related toxicities (eg, diarrhea, skin rash).1
- Preclinical data suggest that tucatinib increases surface HER2 expression.2
- HER2 is overexpressed in multiple cancers, including breast, colorectal, ovarian, lung, gastroesophageal, and bladder.
Mechanism of Action
Stage of Development
Tucatinib has received regulatory approval for specific indications and is being investigated in the tumor types shown below.
HER2+ Breast Cancer
Phase 3 Combination†
Phase 3 Combination*
Phase 3 Combination*
HER2+ Colorectal Cancer
Solid Tumors with HER2 alterations
Phase 2 Combination*

 Back
Back


