CD25V Directed Antibody Drug Conjugate
PF-08046032 is an investigational compound. Its safety and efficacy have not been established
Overview + Rationale
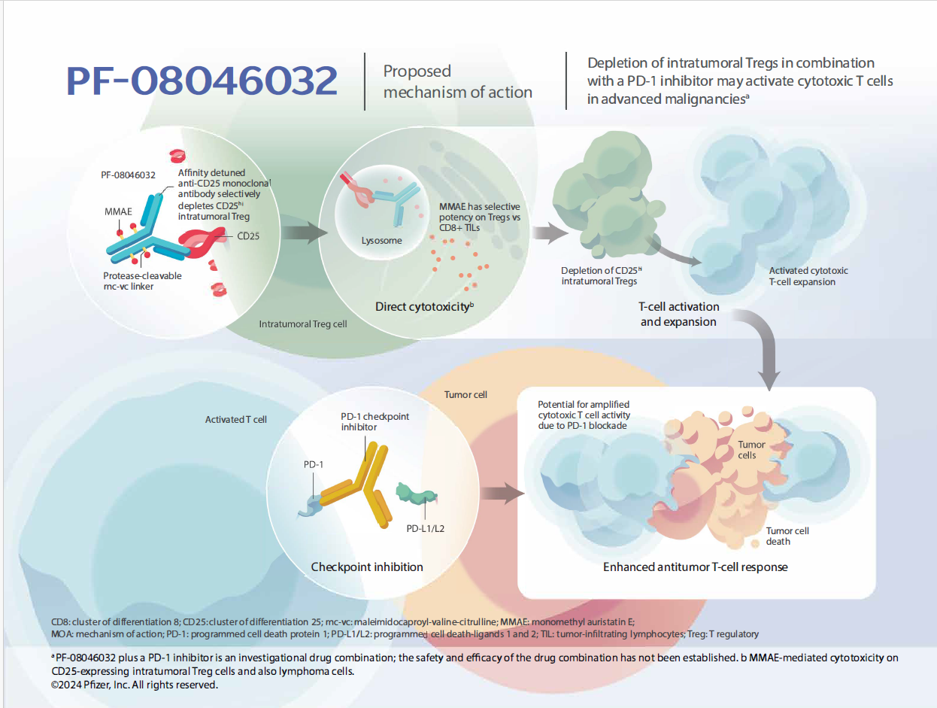
- PF-08046032 is a vedotin antibody-drug conjugate (ADC) engineered to target and potentially deplete immunosuppressive regulatory T cells (Tregs) within the tumor microenvironment (TME) through direct cytotoxicity.
- PF-08046032 is thought to direct delivery of the cytotoxic MMAE (monomethyl auristatin E) vedotin payload to cells expressing CD25 via affinity-tuned binding of the alpha chain of the IL-2 receptor, which is understood to be highly expressed on intratumoral Tregs and lymphoma cells.
- Affinity-tuning can enable targeting of CD25hi intratumoral Tregs while sparing peripheral Tregs.
- PF-08046032 leverages the MMAE-based vedotin drug linker platform
Mechanism of Action

 Back
Back

