Brentuximab vedotin
Overview + Rationale
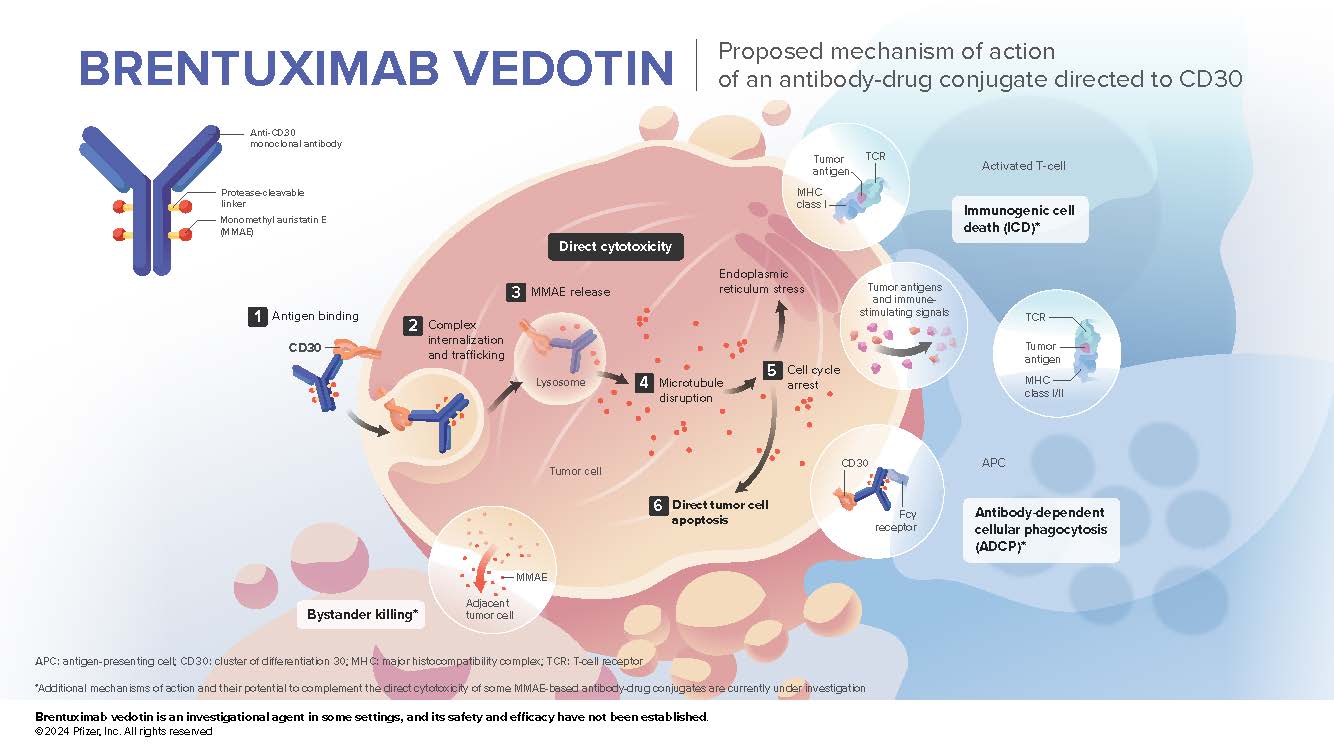
- Brentuximab vedotin (BV) is an antibody-drug conjugate composed of the antibody SGN-30, directed to human CD30,1,2 which is covalently attached to the microtubule-disrupting agent MMAE2,3 via a protease-cleavable mc-vc linker2-4
- CD30 is expressed in many B- and T-cell lymphomas while having limited expression in normal tissue1
- Targeted delivery of MMAE to CD30-expressing tumor cells is the primary mechanism of action of brentuximab vedotin.5 However, the direct cytotoxicity associated with brentuximab vedotin may be complemented by secondary effects,6 including the bystander effect and several important immune-oncology related effects, such as immunogenic cell death7,8,9 and antibody-dependent cellular phagocytosis.10
Partner: Takeda Pharmaceutical Company Limited.
Mechanism of Action
Stage of Development
Brentuximab vedotin is being investigated in combination with other agent(s) in the tumor types shown here. Safety and efficacy for the uses listed below has not been established.

 Back
Back




